Battery Materials
Introduction
Cost-effective energy storage, especially battery technology, is essential in the human transition from petroleum-based energy to a renewable energy economy. Rare and/or expensive battery materials are not suitable for a wide range of practical applications, and an alternative must be found for the currently popular lithium-ion battery technology. As an important supplement to traditional inorganic materials, organic battery materials have been extensively studied in recent years. Compared with inorganic electrode materials containing transition metal elements, organic electrode materials have many advantages. Generally speaking, a battery consists of five major components. An anode, cathode, the current collectors these may sit on, electrolyte and separator, as shown in the figure 1. Organic electrode materials are mainly discussed here.
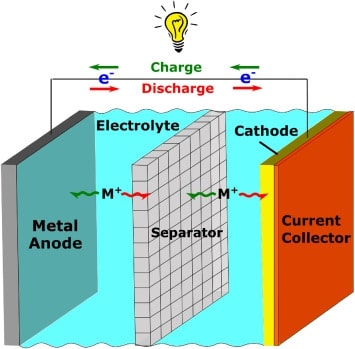 Figure 1. A typical battery format.
Figure 1. A typical battery format.
Classification of organic electrode materials
The history of organic materials can be traced back to the 1960s. Sodium dichloroisocyanurate was first proposed as the cathode material of primary lithium batteries. Since then, various organic materials have been applied to the research of battery active materials. The following will briefly introduce the four most representative organic electrode materials that have been widely studied, namely conductive polymer, organic sulfide, free radical polymer and carbonyl compound.
- Conductive polymer
Conductive polymers usually have good conductivity, which can often obtain better magnification performance when used as battery electrode materials. According to the types of constituent atoms, the conductive polymer molecules that have been widely studied can be mainly divided into three categories: conjugated hydrocarbons, conjugated thioethers and conjugated amines. The representative materials of these three types of polymers are respectively polyacetylene (PAc), polythiophene (PTh) and polyaniline (PAn).
- Organic sulfide polymer
In order to solve the problem of relatively low energy density of conductive polymer electrode materials, people have carried out extensive research on organic sulfide. Organic sulfide usually has high theoretical specific capacity due to its multiple redox active sites. In addition, organic sulfide has the characteristics of low cost, biodegradability and low toxicity, making it one of the most promising organic electrode materials.
- Free radical polymer
Free radical polymers generally contain free radicals in neutral state. Compared with organic sulfur compounds, they have higher magnification performance and stability. In recent years, the research on free radical polymers mainly focuses on aminoxyl radicals. These compounds are bipolar materials, which can be reduced to ammonia oxide anions or oxidized to oxygen ammonia cations. For example, a radical polymer containing 2,2,6,6-tetramethylpiperidine-1-oxyl can be used as a cathode material, which is a stable and inert polymer containing nitrogen and oxygen radicals.
- Carbonyl polymer
Common carbonyl compounds usually have higher capacity than free radical polymers. Common carbonyl compounds are N-type materials, which are generally reduced to free radical anions in the electrochemical process, and then reversibly oxidized to the initial state through alkenylation reaction.
Application
The new materials enable batteries with an unmatched combination of high energy and high power density, providing the technological basis for the realization of portable electronics, power tools and hybrid/all-electric vehicles. The high energy efficiency of battery materials may also enable their use in a variety of grid applications, including improving the quality of energy obtained from wind, solar, geothermal and other renewable sources, thereby facilitating their wider use and creating an energy sustainable economy.
Reference
- Borah, R., Hughson, F. R., Johnston, J., & Nann, T. On battery materials and methods. Materials Today Advances, 2020, 6, 100046.








![4-Methoxy-2,2,6,6-tetramethylpiperidine 1-OxylFree Radical [Catalyst for Oxidation]](https://resource.bocsci.com/structure/95407-69-5.gif)