Vapor Deposition Precursors
Introduction
The deposition of thin film materials from the vapor phase through a chemical reaction is known as chemical vapor deposition (CVD). In this process, the choice of precursor is crucial. To be of greatest utility for CVD, a precursor should: (1) have sufficient vapor pressure to allow transport to the growth surface; (2) undergo facile decomposition to the desired product at an acceptable temperature, and (3) have no association or reaction in the gas phase.
Characteristic
Due to the special requirements on the properties of the target films, a wide variety of precursors have emerged, and the chemistry of the precursors has been greatly developed. For example, synthetic chemists have developed metalorganic precursors with greater volatilities and cleaner deposition chemistry, such as liquid source precursors for improved safety and handling, and single source precursors for growth of binary and tertiary compounds with control of the film stoichiometry.
Common ligands in metal-organic precursor compounds includes alkyl groups (methyl, ethyl, etc.), amides (–NH2, –NR2, etc.), nitrates (NO3), alkoxides (–OR such as t-butoxide, i-propoxide, ethoxide, etc.) and β-diketonates, generally in the form M(β-diketonates)n, where n=2, M=Cu, Ba, Pb, Sr, for n=3, M=Al, Cr, Fe, Co, Ga, and for n=4, M=Ti, Zr, Hf, etc. Common β-diketonates used include acetylacetonate (acac), trifluoroacetylacetonate (tfac), hexafluoroacety-lacetonate (hfac), and 2,2,6,6-tetramethyl-3,5-heptanedionate (tmhd or thd). Mixed ligand metal precursors containing alkoxides and β-diketonates are designed to combine the advantages of alkoxides (high vapor pressures and reduced deposition temperatures) with the improved hydrolysis stability offered by β-diketonate. Metal nitrates are C, H, and halogen-free sources that can grow high quality dielectric films, but are strongly oxidizing and should not be mixed with readily oxidized materials (organics). Single source precursor refers to a precursor without coordination precursor and typically include a metal precursor combined with the required non-metallic sources.Shown in Table 1 are representative examples of some of the most common precursors used today to deposit thin films for microelectronics, optoelectronics, and superconductivity, and for processes used to deposit transparent conductive coatings, and wear and corrosion resistance coatings.
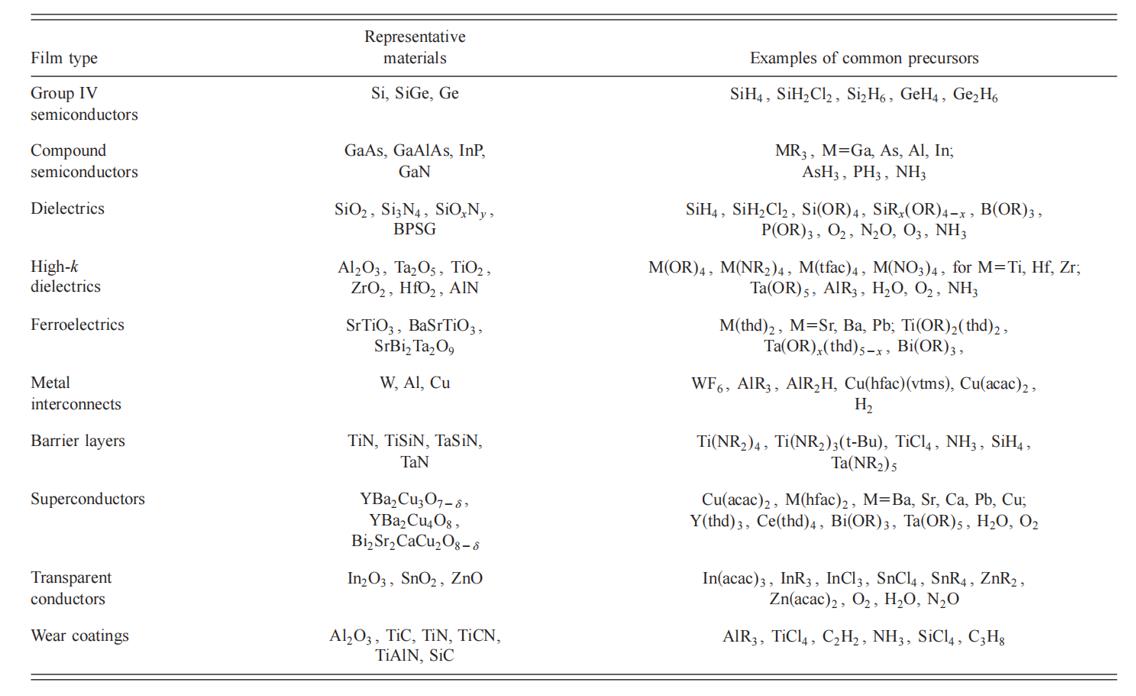 Table 1. Some typical materials deposited by CVD and their uses, and some common precursors utilized to achieve deposition by CVD.[1]
Table 1. Some typical materials deposited by CVD and their uses, and some common precursors utilized to achieve deposition by CVD.[1]
Application
In the past few years, chemical vapor deposition (CVD) has been greatly developed and widely used in various industries, including electronics, optoelectronics and film coating industries. The type and quality of precursors are also gradually improved. The trend of device miniaturization has accelerated the development of deposition technology, including the process, equipment and precursor required for deposition of films with clear characteristics. In addition, the development of alternatives to dielectrics, capacitors, metal interconnects, barrier layers and semiconductor materials used to manufacture devices will lead to greater demand for precursors and processes for depositing these materials, and further use of chemical reaction based deposition methods.
Reference
- Crowell, John E. Chemical methods of thin film deposition: Chemical vapor deposition, atomic layer deposition, and related technologies. Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films, 2003, 21.5: S88-S95.



