OLED and PLED Materials
OLED Structural Principles
Organic electroluminescent device with double-layer sandwich structure is called OLED--Organic Light-Emitting Diode. The structure of a typical OLED device is shown in Fig 1, including:
- Substrate: its material is usually plastic, glass, metal foil.
- Anode: the anode is usually made of indium tin oxide (ITO).
- Hole transport layer: this layer is primarily responsible for transporting holes from the anode, usually an organic material.
- Light-emitting layer: this layer also uses organic materials, and the physical process of OLED light-emitting mainly occurs in this layer.
- Electron transport layer: the main role of this layer is to transport electrons injected from the cathode, also using organic materials.
- Cathode: whether or not the cathode is transparent is determined by the structure of the OLED device. Commonly used cathode materials are aluminum, silver, gold and copper alloys, etc., and which cathode material to use is usually determined according to the type of OLED. The main function of the cathode is to inject electrons.
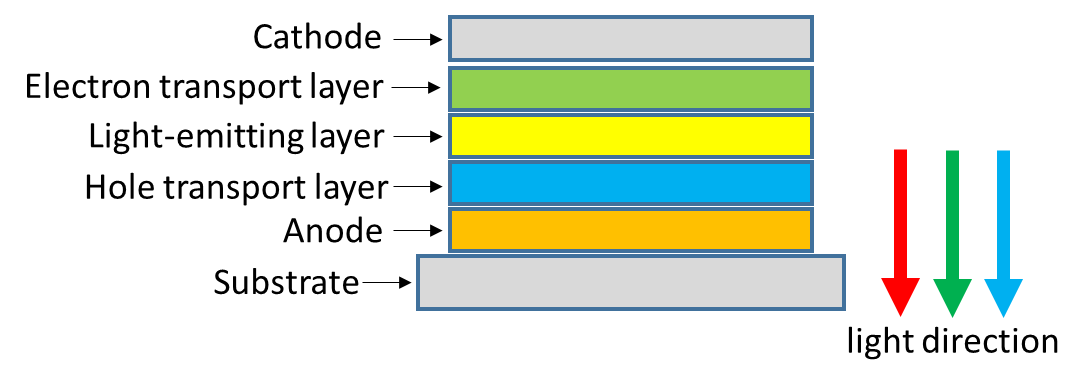 Fig 1. OLED device structure
Fig 1. OLED device structure
OLED Classification and Features
Commonly used host materials for OLEDs include fluorescent materials and phosphorescent materials. In recent years, researchers have developed a new generation of light-emitting materials by adjusting the energy changes of the triplet excited state and the singlet excited state to improve the exciton utilization rate. A typical new generation of luminescent materials is thermally activated delayed-fluorescence (thermally activated delayed-fluorescence, TADF) materials. After holes and electrons recombine to generate excitons, two excited state forms, singlet state and triplet state, will be generated due to the different electron spin symmetry. Among them, the singlet excited states (S1, S2...Sx) are generated by non-spin-symmetric ground state electrons, and a series of high-energy levels of Sx are quickly relaxed to the lowest energy level of S1, and then release energy back to the ground state S0 in the form of fluorescence; Similarly, triplet excited states (T1, T2…Ty) are formed by spin-symmetric ground-state electrons, and the higher-energy level Ty will relax to the lowest-energy level T1, and finally release energy in the form of phosphorescence, as shown in Fig 2(a). According to the spin statistics theory in the coupling law of quantum mechanics, the ratio of spin singlet excitons to spin triplet excitons is about 1:3. The design principle of TADF materials is to form a small energy gap (ΔEST) between S1 and T1 through molecular design, so that triplet excitons can achieve efficient reverse intersystem crossing from T1 to S1 under certain thermal excitation (RISC). as shown in Fig 2(b).
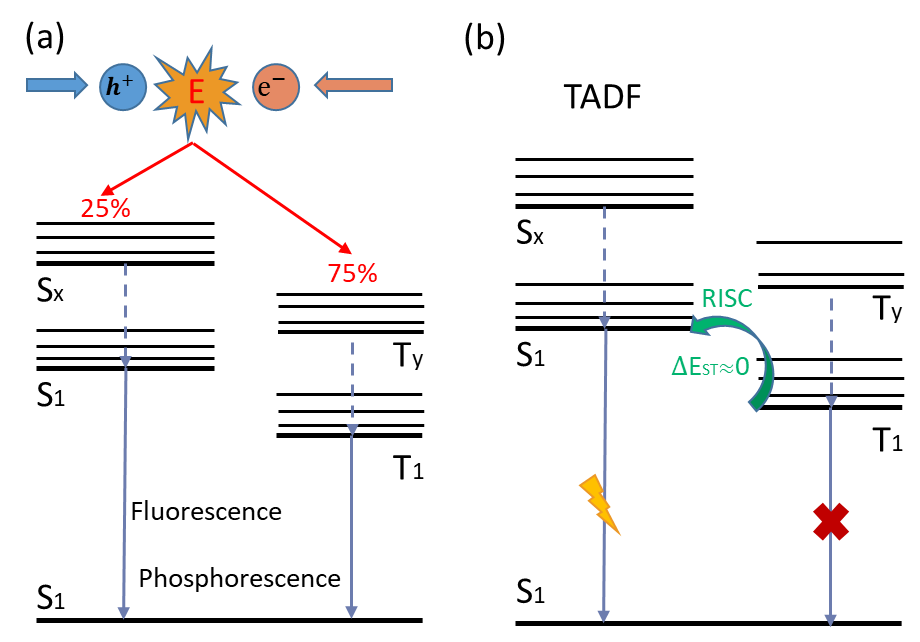 Fig 2. luminescent mechanisms for different organic electroluminescent materials (Xing, 2020)
Fig 2. luminescent mechanisms for different organic electroluminescent materials (Xing, 2020)
The materials that make up OLEDs are mainly organic substances, which can be divided according to the types of organic substances. One is small molecules and the other is macromolecules. Fell reported a series of red light small molecule materials (Fig 3), each of which used 4,7-bis(thiophen-2-yl)benzo[C][1,2,5] Thiadiazole as the core, monofluorene or difluorene to modify the end group, and increase the solubility of the material through the alkyl chain of fluorene, and finally capped with trimethylsilyl, triphenylamine or benzofuran.
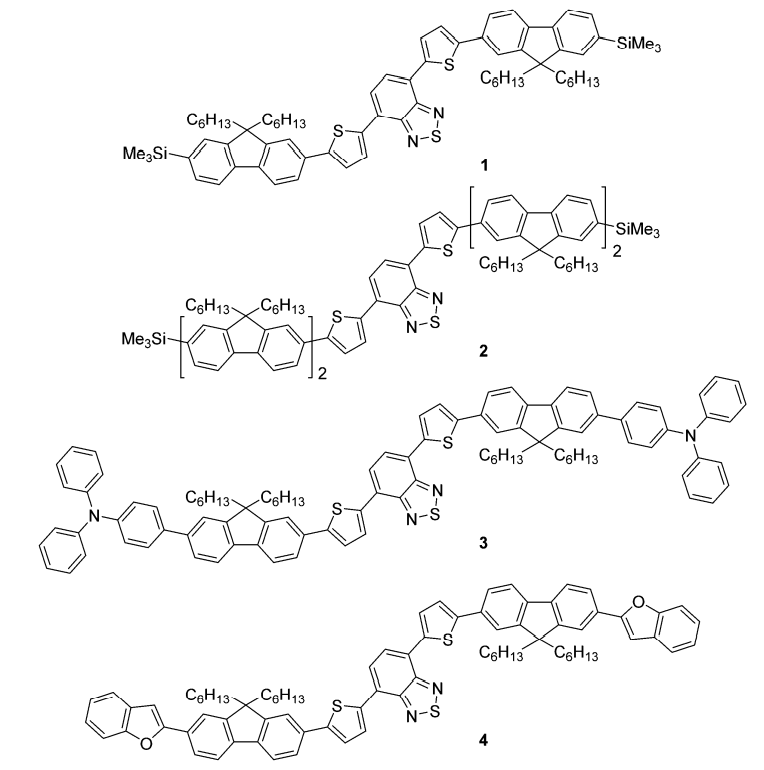 Fig 3. Chemical Structure of OLED small molecule materials
Fig 3. Chemical Structure of OLED small molecule materials
Compared with organic small molecule materials, polymer materials are easy to process and modify, more suitable for OLED technology, and have broad application prospects. Fluorene derivatives have wide band gaps, easy processing, strong fluorescence quantum efficiency and spectral stability, and are the most commonly used blue light conjugated polymerization units. Polyfluorene is a p-type material, dominated by hole transport, and the electron and hole transport is unbalanced. It usually needs to be modified by side chains or end groups to improve, such as introducing electron transport groups, end capping to block hole transport, etc. A polymer (the molecular formula is shown in Fig 4(1)) was reported that was obtained by introducing S,S-dioxygen-dibenzothiophene (SO) into the main chain of polyfluorene, and using triphenylamine (TA) at the same time capping to inhibit intramolecular charge transfer and enhance luminescence efficiency. Pendant fluorene polymers of carbazole and oxadiazole (PFCzOxd-co-PCzs and PFCzOxd-co-PPTZs, the molecular formula is shown in Fig 4(2)) were synthesized and characterized. Incorporation of carbazole or thiazine hole-transporting units in the fluorene framework improves the brightness and luminous efficiency of EL devices.
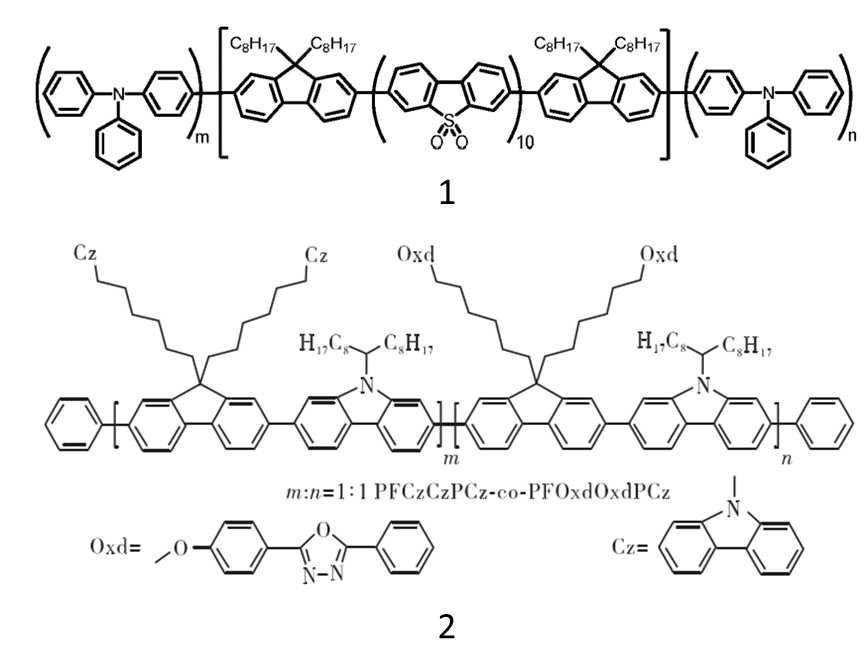 Fig 4. Chemical Structure of OLED Polymer Materials
Fig 4. Chemical Structure of OLED Polymer Materials
References
- Xing L J, Zhu Z L, He J, et al. Chemical Engineering Journal[J], 2020: 127748.
- Fell V, Findlay N J, Breig B, et al. Effect of end group functionalisation of small molecules featuring the fluorene–thiophene–benzothiadiazole motif as emitters in solution-processed red and orange organic light-emitting diodes[J]. Journal of Materials Chemistry C, 2019.
- Hu L, Liang J, Zhong W, et al. Improving the electroluminescence performance of blue light-emitting poly(fluorene-co-dibenzothiophene-S,S-dioxide) by tuning the intra-molecular charge transfer effects and temperature-induced orientation of the emissive layer structure[J]. Journal of Materials Chemistry C, 2019.






