Molecular Conductors
Introduction
Molecular conductor is short for conductive molecular crystal, in which the conducting element is not a metal atom, but a molecule, usually a planar organic π-conjugated molecule. From the point of view of material types, among the molecular conductors with high electrical conductivity that have been synthesized so far, TTF-based (tetrathiafulvalene) (see Figure 1) and its derivative-type molecular conductors are the most intensively studied category. It can act either as an electron donor to form charge-transfer complexes or charge-transfer salts, or as a single-component molecular conductor with good conductive property.
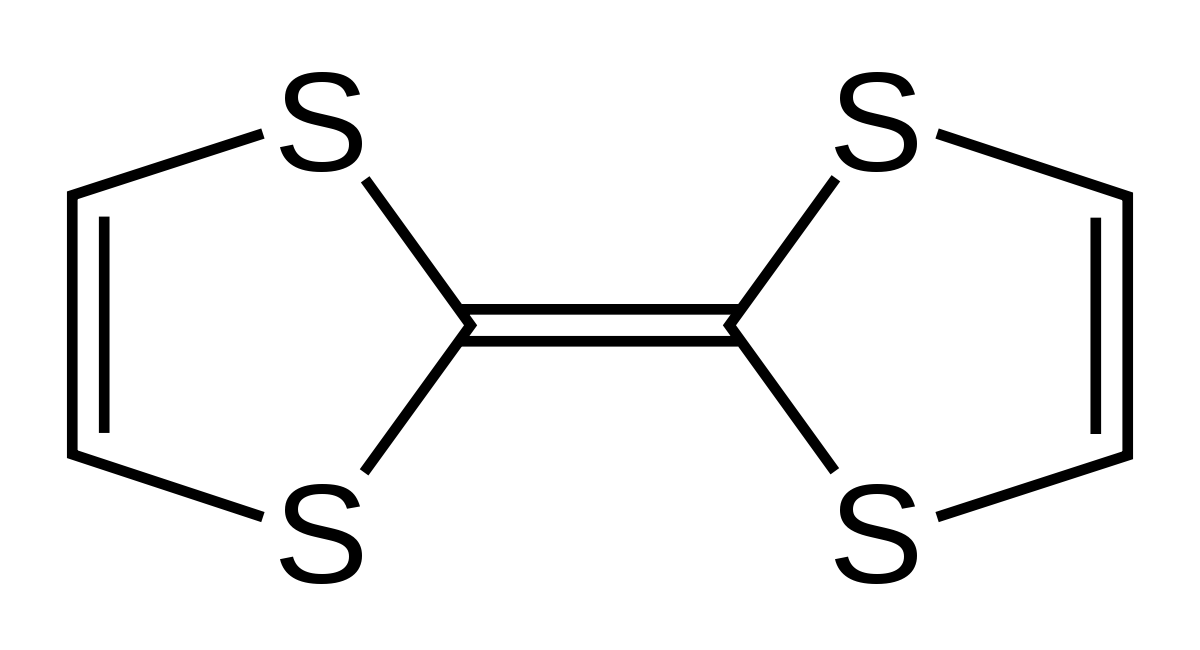 Figure 1. Structural formula of the electron donor TTF
Figure 1. Structural formula of the electron donor TTF
First generation TTF-type electron donor and corresponding molecular conductor
Since the first charge transfer complex TTF-TCNQ was produced by TTF and TCNQ (7,7,8,8-tetracyano-p-quinodimethane), about one thousand compounds of TTF type have been synthesized so far. They can be used not only as electron donors in molecular conductors but also as functional groups in materials chemistry. TTF is a 14π electron aromatic ring system and a strong electron donor system, the synthesis of electron donors often revolves around TTF. TTF has two pairs of reversible redox peaks in solution, this means that it readily gives one or two electrons to form TTF+, TTF2+. The derivatives of TTF may form ordered compact stacks through intermolecular π-π interactions or perhaps side group-side group (S-S) short-range interactions.
Second generation TTF-type electron donor and corresponding molecular conductor
- BEDT-TTF type molecular conductor
The new TTF-type electron donors can be generated by expanding the backbone of TTF with an expanded conjugation surface. The most successful one is the bis(ethylenedithio)-tetrathiafulvalene, abbreviated as BEDT-TTF or ET. The electron donor BEDT-TTF possesses double the number of S atoms and an extended conjugated system compared to TTF, which allows the formed charge transfer salts to exhibit a two-dimensional conductive character. A large number of molecular conductors and superconductors based on BEDT-TTF have been synthesized. The research on BEDT-TTF superconductors was a heyday in the field of molecular conductors.
- TTP type molecular conductor
The chemical formula of TTP (Tetrathiapentalene) is C4H4S4, which has two less carbon atoms than TTF, and it is a structural unit that has been frequently seen in the field of molecular conductors in recent years. A wide range of charge transfer salts based on BDT-TTP (2,5-bis(1,3-dithiol-2-ylidene)-1,3,4,6-tetrathiapentalene), such as β-(BDT-TTP)2X [X= ClO4-, BF4-, ReO4-, PF6-……], exhibit metallic conductivity.
Third generation TTF type (large TTF type) electron donor and corresponding molecular conductor
Based on BDT-TTP, the researchers continued to extend the π-conjugation system by increasing the number of sulfur group atoms, which increased the planarity of the molecule. The increase of intermolecular S-S interactions and π-π interactions is expected to enable molecules exhibit good charge transfer properties, and moreover, is expected to enable the electron donor itself acts as a molecular conductor and has excellent conductive properties, i.e., a single-component molecular conductor. For example, a large TTF-type electron donor containing 12 sulfur group atoms has the structural feature that two TTFs are thickly grouped together and continue to expand the sulfur atoms at the periphery, forming an electron donor with open or closed end groups.
Application
High conductivity and superconducting temperature have been the pursued goals during the development of molecular conductors, which also provide a wide application space for molecular conductors. For example, the incorporation of five- or six-membered rings, such as thiophene or benzene, on TTF parent forms a large π-conjugated structure, which improves the stacking of crystals and is applied to field-effect transistors to increase the field-effect mobility. Or TTF derivatives are assembled into monolayer or multilayer conductive Langmuir-Blodgett films, which can also be prepared as conductive polymers.
Reference
- Kobayashi, Hayao, Akiko Kobayashi, and Hiroyuki Tajima. Studies on molecular conductors: From organic semiconductors to molecular metals and superconductors. Chemistry–An Asian Journal, 2011, 6.7: 1688-1704.



![4,5,9,10-Tetrabromo-2,7-dioctylbenzo[lmn][3,8]phenanthroline-1,3,6,8-tetraone](/images/logo-g.svg)





![2-Bromospiro[fluorene-9,9'-xanthene]](https://resource.bocsci.com/structure/899422-06-1.gif)









