Carboranes
Introduction
Carboranes are a kind of polyhedral boron carbon cluster compounds, which are different from the classical organic boron compounds in the following two aspects: 1) There are 3-6 different boron atoms around the carbon atoms in the carborane skeleton; 2) The electronic structure of the delocalized covalent bond makes carborane have three-dimensional aromaticity and relatively stable chemical properties.
Structure of carborane
Similar to borane, carborane has four configurations: closo-carborane, nido-carborane, arachno-carborane and hypho-carborane. Nido, arachno and hypho carboranes are obtained by removing one, two and three vertices from the closo-carborane framework, respectively. Among these configurations, closo-carborane has the largest number and the most stable properties. take the most common icosahedron C2B10H12 as an example: each carbon atom is adjacent to at least 4-5 boron atoms; there are three isomers, ortho-, meta- and para- carborane, depending on the position of the carbon atom in the backbone. The first two molecules have C2v symmetry, while the latter has D5d symmetry. Ortho carborane can rearrange in an inert gas atmosphere at 400-500 ℃ to form meta carborane; continue to increase the temperature to 600-700 ℃ to obtain para carborane (Fig. 1).
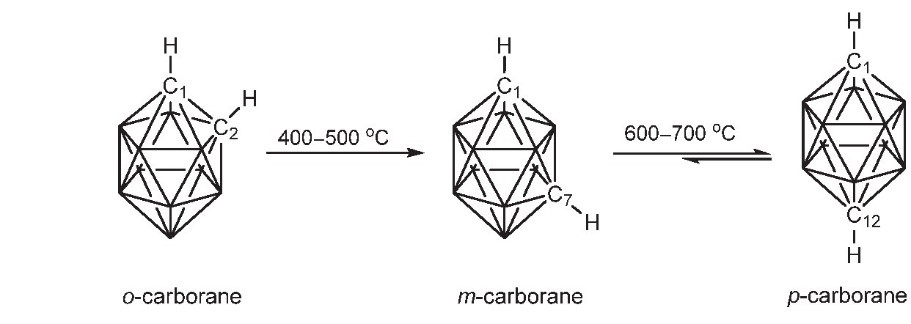 Fig.1 Three Isomers of C2B10H12 and Their Rearrangement Reaction[1]
Fig.1 Three Isomers of C2B10H12 and Their Rearrangement Reaction[1]
The total number of valence electrons in the neutral closo-carborane C2B10H12 molecule is 50, The total number of σ electrons used by C-H and B-H bonds is 24, and the total number of skeleton electrons is 26, occupying 13 bonding orbitals. Research on reaction properties show that it has three-dimensional aromaticity.
Properties of carborane
Carborane has good thermal stability and chemical stability. However, in the presence of strong Lewis alkali such as alcohols, amines, fluoride, carborane undergoes boron cage collapse and removes a boron top to generate a well water-soluble monovalent anion of nido-carborane (e.g. nido-7,8-C2B9H12-). It further reacts with strong alkali to generate divalent anions (similar to cyclopentadienyl anions C5H5-), which can be used to synthesize metal carboranes and B(3)-substituted carboranes. The stability of carborane makes the cage structure not be destroyed when substitution occurs on carbon and boron atoms. Carbon atoms with relatively large electronegativity on molecular cages make carborane have special chemical reactivity. On the one hand, the strong electron absorption of the carborane cage makes the H on the C-H bond weakly acidic, and a series of functionalized carborane derivatives can be prepared by reacting with alkyl lithium reagents, On the other hand, the slightly positive boron atom adjacent to the carbon atom can lead to nucleophilic substitution reaction, while the hydrogen atom directly connected to the boron atom is easy to attack the electrophilic reagent and cause electrophilic substitution reaction.
Application
In the 1990s, carborane and its derivatives showed certain application prospects in nanoscale molecular engineering, catalysts, metal recovery of radioactive nuclear fuel and many other fields. As basic research progressed, siloxane polymers containing carborane have shown commercial value due to their chemical stability. For example, metal carborane can be used as catalyst in hydrogenation reaction, carborane derivatives attached to antibodies can be used for boron neutron capture therapy. In addition, the unique structure of carborane give it potential application in the development of new materials with optical, electrical and magnetic properties.
Reference
- Ochi, Junki; TANAKA, Kazuo; CHUJO, Yoshiki. Recent progress in the development of solid‐state luminescent o‐carboranes with stimuli responsivity. Angewandte Chemie International Edition, 2020, 59.25: 9841-9855.


