Substrates and Electrode Materials
Introduction
The current pursuit of portable electronic products has largely stimulated the improvement of electrical energy storage/conversion efficiency, and has placed new demands on electrode materials and substrates. Therefore, the choice of electrode material and substrate is critical for electrochemical energy storage/conversion yields, which possess a significant influence on the kinetics and thermodynamics of electron transfer and determine the success or failure of the conversion. In specific, the various part of energy storage/conversion system will be introduced in terms of conventional devices, including electrode materials, materials for surface modification and substrates.
Electrode Materials
The electrodes can be classified in terms of material types, including metallic, carbonaceous, and polymeric materials.
Despite their weight and bulk disadvantages, metals are the most direct choice for electrode substrates due to their commercial availability and relatively low cost. Conductivity and potential stability are the two basic criteria when choosing metals as electrode substrates. Good electrical conductivity ensures low internal resistance and fast ion and electron transport in the system, and potential stability means that the metal should not undergo chemical and/or electrochemical reactions during charging and discharging.
Carbon nanomaterials have been favored due to their excellent properties. There are various types of carbon nanomaterials, mainly including freestanding carbon nanomaterials, carbonized materials, carbon cloth-based and electrospun carbon nanofiber materials. Among the freestanding carbon nanomaterials, carbon nanotubes (CNTs) and graphene are representative carbon nanomaterials with excellent mechanical strength, high electrical conductivity, low density, and large specific surface area.
In contrast to metals and carbon materials, most polymers are inherently non-conductive and therefore cannot be used directly as electrodes in electrochemical energy devices. However, polymers offer the distinct advantages of good mechanical flexibility, low density, ease of processing and low cost. In addition, some redox-active conductive polymers (e.g., PANi, Ppy, PEDOT) and organic dye molecules have recently been extensively investigated for flexible energy devices, and they can be fabricated as stand-alone thin-film flexible electrodes.
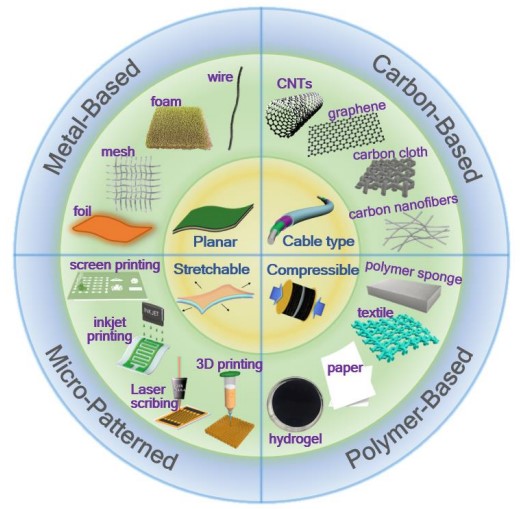 Fig. 1. Schematic of the electrode materials/substrates categorized into four types including metal-based, carbon-based, polymer-based, and micro-patterned flexible electrodes[1].
Fig. 1. Schematic of the electrode materials/substrates categorized into four types including metal-based, carbon-based, polymer-based, and micro-patterned flexible electrodes[1].
Materials for Surface Modification
Modification of electrode materials surfaces to aid catalytic reactions and reduce the overpotential for electron transfer is a proven technique, particularly in energy-related applications such as hydrogen evolution reaction, oxygen evolution, and CO2 reduction. For synthetic applications, immobilization or tethering of the electron transfer mediator to the electrode surface, either covalently or non-covalently, has been shown to improve the efficiency of the reaction. For example, oxidation produces a high density of surface carboxyl groups that can form amide bonds, or single-electron reduction of diazo cations produces aromatic radicals that readily bind to graphite electrodes.
Substrates
As an important part of the energy storage/conversion system, the substrate assumes the role of support and interaction with the electrodes. For example, strontium titanate can be used not only as an excellent substrate for the epitaxial growth of high-temperature superconductors and many oxide based thin films, but also for electrode substrates. It is particularly well known as a substrate for growth at the lanthanum aluminate-strontium titanate interface, doped with niobium to make it conductive, and is one of the only commercially available conductive single crystal substrates for perovskite growth.
Application
Currently, efforts are underway to develop new energy storage and conversion devices for ultra-thin, lightweight, flexible, comfortable, durable, and secure wearable applications. Therefore, the development of properties for electrode materials and substrates is essential. Breakthroughs have been made in device configuration design (e.g., one-dimensional fiber-like devices), new battery system development (e.g., supercapacitors, Li-S cells, K/Na ion batteries, zinc ion batteries, and metal-air batteries), and packaging technology optimization, all of which have benefited from the advancement of battery materials and substrates.
Reference
- Shang, Kezheng, et al. An Overview of Flexible Electrode Materials/Substrates for Flexible Electrochemical Energy Storage/Conversion Devices. European Journal of Inorganic Chemistry, 2021, 2021.7: 606-619.



