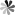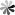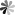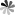Perovskite Solar Cell (PSC) Materials
Introduction
Perovskite Solar Cell (PSC) is generally composed of fluorine doped SnO2 (fluorine-tin-oxide, FTO) conductive glass, electron transport layer, photosensitive layer, hole transport layer, metal electrode and other parts. The typical structure of PSC is shown in the Fig. 1. PSC materials include the materials required for the above parts, among which the most primary are hole transport materials, photosensitive materials and electron transport materials.
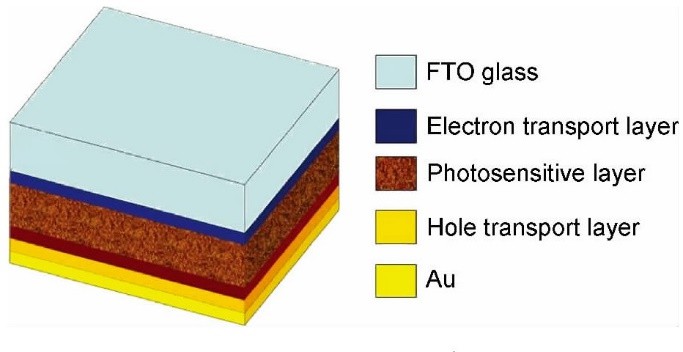 Fig.1 Schematic structure of PSC
Fig.1 Schematic structure of PSC
Photosensitive Materials
The structure of the photosensitive material of the PSC is an organic-inorganic hybrid perovskite structure, with the chemical formula as follow: ABX3(A:CH3NH3+, CH (NH2)2+; B:Pb2+, Sn2+; X:l-, Br-, Cl-), which is a typical perovskite (CaTiO3) crystal structure. In ABX3 crystal, BX6 forms a regular octahedron, and BX6 is connected by sharing vertex X to form a three-dimensional skeleton, and A is embedded in the octahedral gap to stabilize the crystal structure. CH3NH3Pbl3, the most widely used light absorbing material in PSC, is a direct band gap semiconductor with a band gap of about 1.5eV, close to the theoretical optimal band gap of single junction solar cells. The metal organic halide ABX3 perovskite material can easily adjust the band gap by changing the combination of A, B and X components, taking CH3NH3PbX3 as an example, with the halogen ion Cl-, Br- and I- radius gradually increases, the band gap of CH3NH3PbX3 decreases in turn. The band gap of CH3NH3PbCI3, CH3NH3PbBr3 and CH3NH3PbI3 is 3.11 eV, 2.22 eV, and 1.51 eV, respectively.
Hole Transport Materials
The most widely used organic small molecule hole transport material in PSC is Spiro-OMe TAD (2,2',7,7'-tetrakis(N,N-di-p-methoxyphenylamine)-9,9'-spirobifluorene). It should be pointed out that the use of Spiro-OMe TAD as a hole transport material requires doping lithium salts and pyridine derivatives to improve hole mobility. Although Spiro-OMe TAD has high photoelectric conversion efficiency, its preparation is difficult and expensive. Therefore, in order to reduce the cost of batteries, developing low-cost alternatives to Spiro-OMe has become the focus of current hole transport material research. For example, Poly(3-hexylthiophene-2,5-diyl) (P3HT) is the most commonly used electron donor material for organic solar cells. When it is used as a hole transport material in PSC, the photoelectric conversion efficiency is also very significant. Inorganic hole transport materials mainly include Cul, CuSCN, NiO, etc., which have the advantages of high hole mobility and low cost.
Electron Transport Materials
The commonly used electron transport material in PSC is TiO2. Generally, a dense TiO2 layer is prepared on fluorine doped tin oxide (FTO) or indium tin oxide (ITO), whose main function is to reduce the potential barrier in electron transport and block the recombination of the conduction band electrons of the electrode and the holes on the valence band of perovskite. If Al is used as cathode, fullerene derivative [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) is usually used as electron transmission material.
Application
In just a few years, PSC has developed rapidly. Its photoelectric conversion efficiency has reached 20%, surpassing that of amorphous silicon and approaching that of polycrystalline silicon. It has become one of the strongest competitors in the photovoltaic field, which cannot be separated from the support of excellent materials. The choice of PSC materials determines whether PSC has the advantages of high conversion efficiency, low preparation cost, and environmental friendliness. At present, PSC materials have been widely applied in the research and development of various new perovskite solar cells, which is expected to make PSC an independent clean energy for wearable devices.
Reference
- Basumatary, Pilik, and Pratima Agarwal. A short review on progress in PSC. Materials Research Bulletin 2022, 149: 111700.


![Poly[2,1,3-benzothiadiazole-4,7-diyl(9,9-dioctyl-9H-fluorene-2,7-diyl)]](https://resource.bocsci.com/structure/210347-52-7.gif)



![5-Azoniaspiro[4.4]nonane Chloride](/images/logo-g.svg)